What is congenital athymia?
Congenital athymia is a rare immune condition that requires children and often their families to live in strict isolation1
Congenital athymia is a primary immunodeficiency, which is a type of immune condition that occurs when part of the immune system is missing. Children with congenital athymia are born without a thymus.2,3
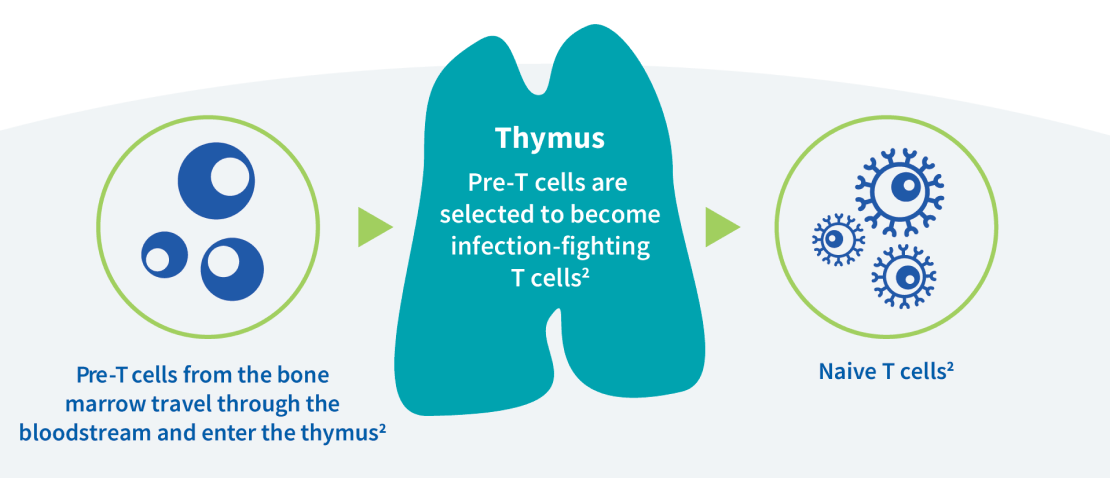
The thymus is an organ that sits on top of the heart and plays an important role in how the immune system works. Without one, these children can face life-threatening infections because they are unable to produce enough naive T cells—white blood cells that help fight off the organisms that cause infections.2,4
Approximately 17 to 24 infants are born with congenital athymia in the US each year.1
Diagnosing congenital athymia
Congenital athymia is often detected through newborn screening for severe combined immunodeficiency (SCID), a test that is required in all 50 states in the US.2
While these two conditions are not the same, the test for SCID will likely indicate to your child’s healthcare provider that further testing and examination are needed. After a positive screening result, an immunologist will use a laboratory technique called flow cytometry to verify low T cells and possibly lead to a diagnosis of congenital athymia.2
The sooner congenital athymia is identified, the sooner isolation and infection prevention measures can begin.2 Explore supportive care
Congenital athymia is associated with other genetic conditions
Congenital athymia has previously been referred to as complete DiGeorge anomaly, but it is now known to be associated with multiple genetic conditions, congenital syndromes, and environmental exposures. However, for some children there is no known cause. Prenatal testing may detect genetic abnormalities that are associated with congenital athymia, but congenital athymia is usually not detected until birth. Some of these associated conditions include2,5-7:
Complete DiGeorge Syndrome
Complete DiGeorge syndrome often occurs when part of chromosome 22 is missing (22q11.2 deletion syndrome). This may result in a compromised immune system as well as potential heart defects, developmental delay, and hearing loss, among other conditions.2
CHARGE Syndrome
Often associated with a mutation in a gene known as CHD7, this consists of medical and physical conditions that differ from child to child. The CHARGE acronym comes from the first letter of some of the more common features seen in children2:
- CColoboma, or defects in the way the eye forms
- HHeart defects
- AAtresia of the choanae, or blocked nasal passages
- RRetardation of growth and development
- GGenital underdevelopment
- EEar abnormalities
FOXN1 Deficiency
This condition affects the production of critical proteins, which may result in a severely compromised immune system and problems with the growth of hair and nails.2
Diabetic Embryopathy
This condition may be associated with altered fetal thymus size and other congenital abnormalities. Abnormal thymic development has been seen in infants of diabetic mothers.2
Children with congenital athymia require special care.2
RETHYMIC is the first and only FDA-approved tissue-based treatment for congenital athymia engineered to help children develop an immune system sufficient to fight infections.8,9
Enzyvant CONNECT provides support and resources to children with congenital athymia and their caregivers.
Indication and Important Safety Information
Important Safety Information
Infection Control: Immune reconstitution sufficient to protect from infection is unlikely to develop prior to 6-12 months after treatment with RETHYMIC. Immune reconstitution is needed for the body to produce cells in the immune system to fight infection. Your child’s doctor should advise you of infection control measures which should be followed immediately after treatment and until the immune system starts working at a sufficient level. Monitor your child closely for signs of infection, including fever. Your child should be maintained on immunoglobulin replacement and prophylactic antimicrobials until certain criteria are met as determined by your doctor.
Graft versus Host Disease (GVHD): RETHYMIC may cause or make pre-existing GVHD worse. Your child will be monitored for GVHD and treated if needed. Symptoms of GVHD may include fever, rash, enlarged lymph nodes, inflammation of the gastrointestinal system and/or diarrhea.
Autoimmune Disorders: Autoimmune-related adverse events occurred in patients treated with RETHYMIC. These events included: low platelets, low white blood cells, protein in urine, low red blood cells, hair loss, poor thyroid function, inflammation of liver, inflammation of the joints, inflammation of the spinal cord, loss of pigment in the skin, eyes and hair, overactive thyroid function, and loss of function of the ovaries. Your doctor will monitor your child regularly including performing blood tests.
Kidney Disease: Treatment with RETHYMIC is a risk factor for death in patients with pre-existing kidney disease.
Cytomegalovirus (CMV) Infection: In clinical studies with RETHYMIC, 4 out of 4 patients with pre-existing CMV infection prior to the implantation with RETHYMIC died. Talk to your doctor about the benefits/risks of treatment if your child has pre-existing CMV infection.
Cancer: Due to your child’s weakened immune system, there is increased risk of developing certain cancers. Your child’s doctor will monitor your child through testing for Epstein-Barr virus (EBV) and cytomegalovirus (CMV), which are two viruses that can cause cancer.
Transmission of Serious Infections: Because RETHYMIC is made from human tissue, and animal products are used in the manufacturing process, transmission of infectious diseases may occur.
Vaccinations: Your child should not receive any vaccinations until he or she has met certain requirements set by your doctor. Talk to your child’s doctor prior to any vaccinations.
Anti-HLA Antibodies: Prior to receiving RETHYMIC your child will be tested for HLA antibodies, which are proteins that may be present in your child’s blood. If your child has these antibodies, he/she will need to receive RETHYMIC from a donor that does not express those HLA proteins.
HLA Typing: If your child has received a hematopoietic cell transplantation (HCT) or a solid organ transplant, they will have a test to look for specific antibodies that could interfere with the effect of RETHYMIC. If they are present, then it will be necessary to receive RETHYMIC from a certain group of donors that do not have these proteins.
Deaths: 105 children participated in the clinical studies of RETHYMIC. 29 of the patients died, including 23 in the first year after implantation of RETHYMIC.
What are the most common side effects with RETHYMIC?
The most common side effects with RETHYMIC are hypertension (high blood pressure), cytokine release syndrome, rash, hypomagnesemia (low magnesium), renal impairment / failure (decrease of kidney function), thrombocytopenia (low platelets), and graft versus host disease.
These are not all of the possible side effects of RETHYMIC. Talk to your child’s doctor about any side effect that bothers your child or does not go away.
You are encouraged to report side effects to the FDA at 1-800-FDA-1088 or www.fda.gov/safety/medwatch.
Indication
RETHYMIC® (allogeneic processed thymus tissue–agdc) is indicated for immune reconstitution in pediatric patients with congenital athymia.
RETHYMIC is not for use in patients who have been diagnosed with severe combined immunodeficiency (SCID).
References: 1. Hsieh EWY, Kim-Chang JJ, Kulke S, Silber A, O’Hara M, Collins C. Defining the clinical, emotional, social, and financial burden of congenital athymia. Adv Ther. 2021;38(8):4271-4288. doi:10.1007/s12325-021-01820-9 2. Collins C, Sharpe E, Silber A, Kulke S, Hsieh EWY. Congenital athymia: genetic etiologies, clinical manifestations, diagnosis, and treatment. J Clin Immunol. 2021;41(5):881-895. doi:10.1007/s10875-021-01059-7 3. Immune Deficiency Foundation. Patient & family handbook for primary immunodeficiency diseases. 6th ed. 2019. 4. NCI Dictionary of Cancer Terms. T cell. National Cancer Institute. Accessed March 11, 2023. https://www.cancer.gov/publications/dictionaries/cancer-terms/def/t-cell 5. Markert ML, Gupton SE, McCarthy EA. Experience with cultured thymus tissue in 105 children. J Allergy Clin Immunol. 2022;149(2):747-757. doi:10.1016/j.jaci.2021.06.028 6. Markert ML. Defects in thymic development. In: Sullivan KE, Stiehm ER, eds. Stiehm’s Immune Deficiencies: Inborn Errors of Immunity. 2nd ed. Elsevier; 2020:357-379. 7. Mustillo PJ, Sullivan KE, Chinn IK, et al. Clinical practice guidelines for the immunological management of chromosome 22q11.2 deletion syndrome and other defects in thymic development. J Clin Immunol. 2023;43(2):247-270. doi:10.1007/s10875-022-01418-y 8. RETHYMIC [package insert]. Marlborough, MA: Sumitomo Pharma America, Inc; 2023. 9. Enzyvant Therapeutics GmbH. Enzyvant receives FDA approval for RETHYMIC® (allogeneic processed thymus tissue-agdc), a one-time regenerative tissue-based therapy for pediatric congenital athymia. Enzyvant Therapeutics, Inc. October 8, 2021. Accessed March 3, 2023. https://enzyvant.com/enzyvant-receives-fda-approval-for-rethymic-allogeneic-processed-thymus-tissue-agdc-a-one-time-regenerative-tissue-based-therapy-for-pediatric-congenital-athymia/