Clinical trial results
RETHYMIC greatly improved survival for patients with congenital athymia1
The efficacy and safety of RETHYMIC were evaluated in 105 pediatric patients across 10 open-label, prospective, single-center clinical trials, including 95 patients in the primary efficacy analysis, with a follow-up of up to 25.5 years.1,2
Patient demographics1
Black
Asian/Pacific Islander
American Indian/Alaskan Native
Multi-race
22
4
2
2
CHARGE syndrome
FOXN1 deficiency
TBX variant
24
2
1
Atypical*
44
*These patients may have had a rash, lymphadenopathy, or oligoclonal cells.1
Survival rates
Survival by Year1
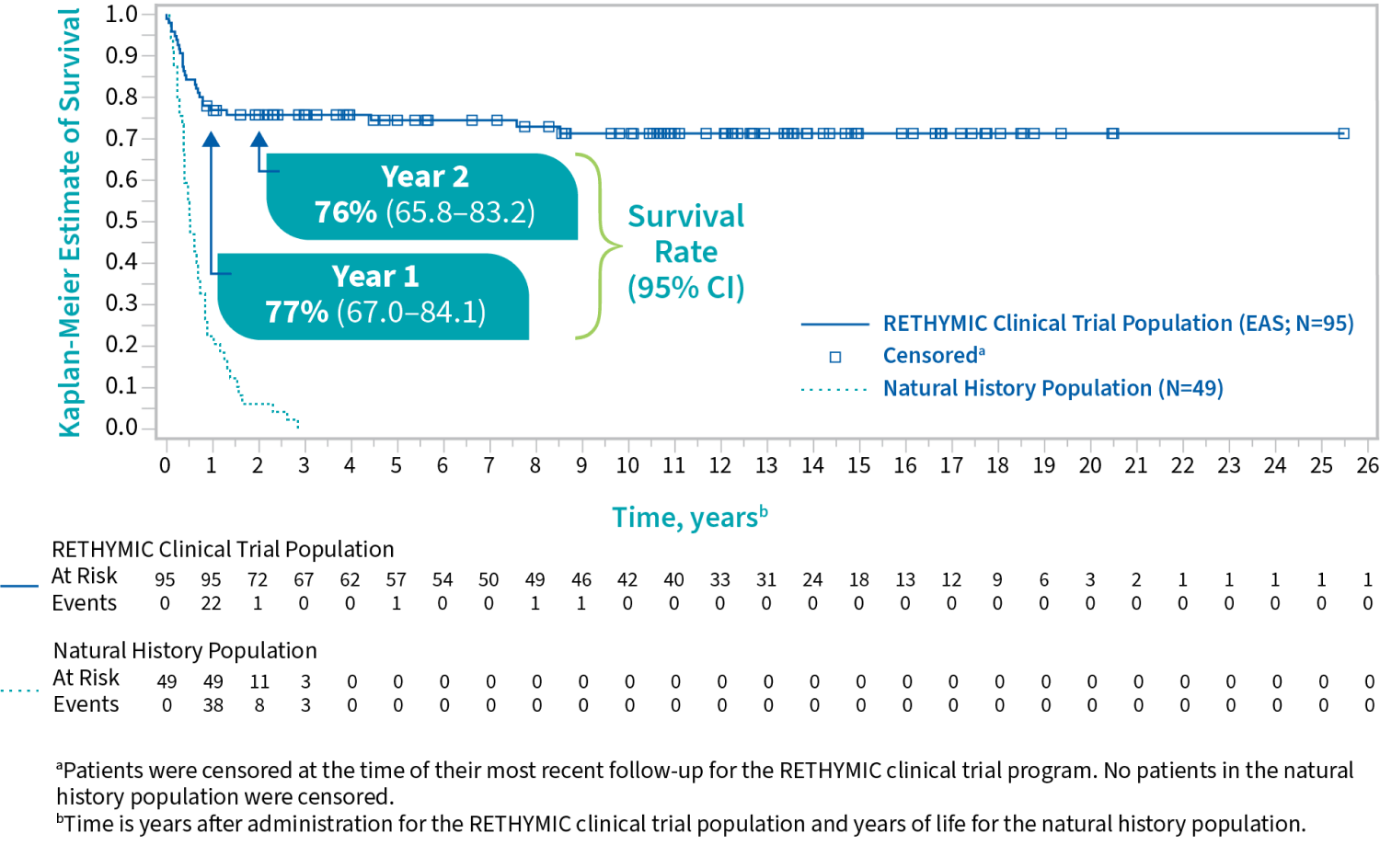
For patients who were alive at 1 year after treatment, the survival rate was
with a median follow-up of 10.7 years1
In a natural history study, congenital athymia patients on supportive care alone typically did not survive beyond 2 to 3 years of age.1
Immune system development
Secondary endpoint: Naive CD4+ and CD8+ T cells reconstituted over the first year following treatment and increased through year 2.1,2
Development of Naive T Cells Following Treatment1,2
(min, max)
Number of subjects
(min, max)
Number of subjects
(0, 38)
63
(0, 46)
60
(0, 653)
62
(0, 163)
53
(1, 751)
42
(0, 304)
37
(33, 858)
26
(6, 275)
26
(min, max)
Number of subjects
(0, 38)
63
(min, max)
Number of subjects
(0, 46)
60
(min, max)
Number of subjects
(0, 653)
62
(min, max)
Number of subjects
(0, 163)
53
(min, max)
Number of subjects
(1, 751)
42
(min, max)
Number of subjects
(0, 304)
37
(min, max)
Number of subjects
(33, 858)
26
(min, max)
Number of subjects
(6, 275)
26
Immune reconstitution sufficient to protect against infection is unlikely to develop prior to 6 to 12 months after treatment, and for some patients, may take up to 2 years.1
Infection reduction
RETHYMIC significantly decreased the rate of infections in the first 2 years after treatment.1
AT 6 TO ≤12 MONTHS AFTER TREATMENT,
fewer patients
experienced an infection event
vs 0 to ≤6 months after treatment (P<0.001)1
At 12 TO ≤24 MONTHS AFTER TREATMENT,
THERE WAS A MEAN DIFFERENCE OF
events per patient
vs 0 to ≤12 months after treatment (P<0.001)1
The safety of RETHYMIC was demonstrated in 105 patients across 10 clinical trials1
The most common (≥10%) adverse reactions related to RETHYMIC were hypertension, cytokine release syndrome, hypomagnesemia, rash, renal impairment/failure, thrombocytopenia, and graft versus host disease (GVHD).1
Adverse reactions occurring in at least 5% of patients in the first 2 years after treatment1
n (%)
All events occurred in association with anti-thymocyte globulin [rabbit] treatment
granuloma skin, rash papular, and urticaria
acute kidney injury, proteinuria, and increased blood creatinine
and immune thrombocytopenic purpura
GVHD-gut, GVHD-skin, and Omenn syndrome
autoimmune hemolytic anemia, Coombs-positive hemolytic anemia, and hemolysis
hypoxia, and respiratory failure
renal tubular acidosis, and decreased blood bicarbonate
and hemorrhagic diarrhea
infantile spasms, and febrile convulsions
Of the 105 patients in the clinical studies, 29 died, including 23 in the first year. The majority of deaths in the first year after receiving RETHYMIC were due to infections (13).1
Unlike a transplant, RETHYMIC is engineered for one patient at a time through a complex process using donor thymus tissue.1,3
See what you should expect after your patients receive treatment with RETHYMIC.
Enzyvant CONNECT provides support and resources for patients with congenital athymia and their caregivers.
Indication and Important Safety Information
Important Safety Information
Immune reconstitution sufficient to protect from infection is unlikely to develop prior to 6-12 months after treatment with RETHYMIC. Given the immunocompromised condition of athymic patients, follow infection control measures until the development of thymic function is established as measured through flow cytometry. Monitor patients closely for signs of infection including fever. If a fever develops, assess the patient by blood and other cultures and treat with antimicrobials as clinically indicated. Patients should be maintained on immunoglobulin replacement therapy until specified criteria are met, and two months after stopping, IgG trough level should be checked. Prior to and after treatment with RETHYMIC, patients should be maintained on Pneumocystis jiroveci pneumonia prophylaxis until specified criteria are met.
RETHYMIC may cause or exacerbate pre-existing graft versus host disease (GVHD). Monitor and treat patients at risk for the development of GVHD. Risk factors for GVHD include atypical complete DiGeorge anomaly phenotype, prior hematopoietic cell transplantation (HCT) and maternal engraftment. GVHD may manifest as fever, rash, lymphadenopathy, elevated bilirubin and liver enzymes, enteritis, and/or diarrhea.
Autoimmune-related adverse events occurred in patients treated with RETHYMIC. These events included: thrombocytopenia, neutropenia, proteinuria, hemolytic anemia, alopecia, hypothyroidism, autoimmune hepatitis, autoimmune arthritis, transverse myelitis, albinism, hyperthyroidism, and ovarian failure. Monitor for the development of autoimmune disorders, including complete blood counts with differential, liver enzymes, serum creatinine, urinalysis, and thyroid function.
Pre-existing renal impairment is a risk factor for death.
In the clinical studies of RETHYMIC, 4 out of 4 patients with pre-existing cytomegalovirus infection died. The benefits/risks of treatment should be considered prior to treating patients with pre-existing CMV infection.
Because of the underlying immune deficiency, patients who receive RETHYMIC may be at risk of developing post-treatment lymphoproliferative disorder. Patients should be monitored for the development of lymphoproliferative disorder.
Transmission of infectious disease may occur because RETHYMIC is derived from human tissue and because product manufacturing includes porcine- and bovine-derived reagents.
Immunizations should not be administered in patients who have received RETHYMIC until immune-function criteria have been met.
All patients should be screened for anti-HLA antibodies prior to receiving RETHYMIC. Patients testing positive for anti-HLA antibodies should receive RETHYMIC from a donor who does not express those HLA alleles. HLA matching is required in patients who have received a prior HCT or a solid organ transplant. Patients who have received a prior HCT are at increased risk of developing GVHD after RETHYMIC if the HCT donor did not fully match the recipient.
Of the 105 patients in clinical studies, 29 patients died, including 23 deaths in the first year (< 365 days) after implantation.
The most common (>10%) adverse events related to RETHYMIC included: hypertension, cytokine release syndrome, rash, hypomagnesemia, renal impairment/failure, thrombocytopenia, and graft versus host disease.
To report suspected adverse reactions, please contact the FDA at 1-800-FDA-1088 or www.fda.gov/safety/medwatch
Indication
RETHYMIC® (allogeneic processed thymus tissue–agdc) is indicated for immune reconstitution in pediatric patients with congenital athymia.
Limitations of Use:
RETHYMIC is not indicated for the treatment of patients with severe combined immunodeficiency (SCID).
References: 1. RETHYMIC [package insert]. Marlborough, MA: Sumitomo Pharma America, Inc; 2023. 2. Markert ML, Gupton SE, McCarthy EA. Experience with cultured thymus tissue in 105 children. J Allergy Clin Immunol. 2022;149(2):747-757. doi:10.1016/j.jaci.2021.06.028 3. Markert ML, McCarthy EA, Gupton SE, Lim AP. Cultured thymus tissue transplantation. In: Sullivan KE, Stiehm ER, eds. Stiehm’s Immune Deficiencies: Inborn Errors of Immunity. 2nd ed. Elsevier; 2020:1229-1239.