Clinical trial results
RETHYMIC greatly improved survival for patients with congenital athymia1
The efficacy and safety of RETHYMIC were evaluated in 105 pediatric patients across 10 open-label, prospective, single-center clinical trials, including 95 patients in the primary efficacy analysis, with a follow-up of up to 25.5 years.1,2
Patient demographics1
Characteristic
Primary Efficacy Analysis (N=95)
Median (range) age at the time of treatment, months
9 (1-36)
Male, %
59
Race, %
White
Black
Asian/Pacific Islander
American Indian/Alaskan Native
Multi-race
70
22
4
2
2
Associated conditions, %
22q11.2 deletion
CHARGE syndrome
FOXN1 deficiency
TBX variant
38
24
2
1
Diagnosed with complete DiGeorge syndrome*, %
Typical
Atypical*
53
44
*These patients may have had a rash, lymphadenopathy, or oligoclonal cells.1
Survival rates
Primary and supportive endpoints: Kaplan-Meier estimated survival rates were 77% (95% CI, 0.670, 0.841) at year 1 and 76% (95% CI, 0.658, 0.832) at year 2.1,2
Survival by Year1
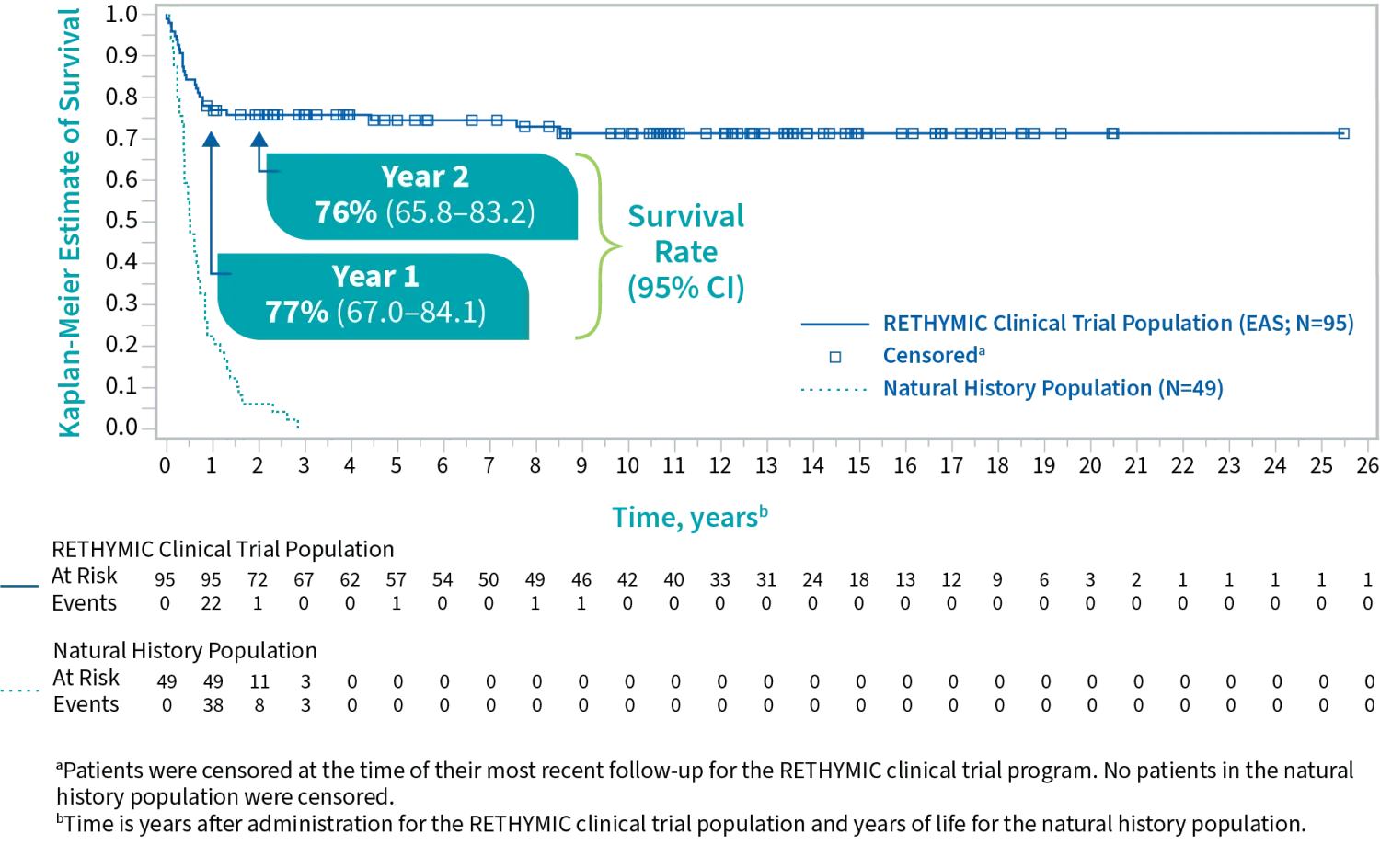
For patients who were alive at 1 year after treatment, the survival rate was
with a median follow-up of 10.7 years1
In a natural history study, congenital athymia patients on supportive care alone typically did not survive beyond 2 to 3 years of age.1
Immune system development
Secondary endpoint: Naive CD4+ and CD8+ T cells reconstituted over the first year following treatment and increased through year 2.1,2
Development of Naive T Cells Following Treatment1,2
Baseline
Month 6
Month 12
Month 24
Median naive CD4+ T cells/mm3(min, max)Number of subjects
1.0 (0, 38)63
42 (0, 653)62
212 (1, 751)42
275 (33, 858)26
Median naive CD8+ T cells/mm3(min, max)Number of subjects
0 (0, 46)60
9 (0, 163)53
58 (0, 304)37
86 (6, 275)26
Baseline
Median naive CD4+ T cells/mm3(min, max)Number of subjects
1.0 (0, 38)63
Median naive CD8+ T cells/mm3(min, max)Number of subjects
0 (0, 46)60
Month 6
Median naive CD4+ T cells/mm3(min, max)Number of subjects
42 (0, 653)62
Median naive CD8+ T cells/mm3(min, max)Number of subjects
9 (0, 163)53
Month 12
Median naive CD4+ T cells/mm3(min, max)Number of subjects
212 (1, 751)42
Median naive CD8+ T cells/mm3(min, max)Number of subjects
58 (0, 304)37
Month 24
Median naive CD4+ T cells/mm3(min, max)Number of subjects
275 (33, 858)26
Median naive CD8+ T cells/mm3(min, max)Number of subjects
86 (6, 275)26
Immune reconstitution sufficient to protect against infection is unlikely to develop prior to 6 to 12 months after treatment, and for some patients, may take up to 2 years.1
Infection reduction
RETHYMIC significantly decreased the rate of infections in the first 2 years after treatment.1
AT 6 TO ≤12 MONTHS AFTER TREATMENT,
fewer patients
experienced an infection event
vs 0 to ≤6 months after treatment (P<0.001)1
AT 12 TO ≤24 MONTHS AFTER TREATMENT,
THERE WAS A MEAN DIFFERENCE OF
events per patient
vs 0 to ≤12 months after treatment (P<0.001)1
The safety of RETHYMIC was demonstrated in 105 patients across 10 clinical trials1
The most common (≥10%) adverse reactions related to RETHYMIC were hypertension, cytokine release syndrome, hypomagnesemia, rash, renal impairment/failure, thrombocytopenia, and graft versus host disease (GVHD).1
Adverse reactions occurring in at least 5% of patients in the first 2 years after treatment1
System organ class
RETHYMIC (N=105)
n (%)
Number of patients with adverse reactions
80 (76)
Hypertension
20 (19)
Cytokine release syndrome
All events occurred in association with anti-thymocyte globulin [rabbit] treatment
19 (18)
Hypomagnesemia
17 (16)
Rash,
granuloma skin, rash papular, and urticaria
16 (15)
Renal impairment/failure,
acute kidney injury, proteinuria, and increased blood creatinine
13 (12)
Thrombocytopenia,
and immune thrombocytopenic purpura
13 (12)
Graft versus host disease,
GVHD-gut, GVHD-skin, and Omenn syndrome
11 (10)
Hemolytic anemia,
autoimmune hemolytic anemia, Coombs-positive hemolytic anemia, and hemolysis
9 (9)
Neutropenia
9 (9)
Respiratory distress,
hypoxia, and respiratory failure
8 (8)
Proteinuria
7 (7)
Pyrexia
6 (6)
Acidosis,
renal tubular acidosis, and decreased blood bicarbonate
6 (6)
Diarrhea,
and hemorrhagic diarrhea
5 (5)
Seizure,
infantile spasms, and febrile convulsions
5 (5)
Of the 105 patients in the clinical studies, 29 died, including 23 in the first year. The majority of deaths in the first year after receiving RETHYMIC were due to infections (13).1
Unlike a transplant, RETHYMIC is engineered for one patient at a time through a complex process using donor thymus tissue.1,3
See what you should expect after your patients receive treatment with RETHYMIC.
Enzyvant CONNECT provides support and resources for patients with congenital athymia and their caregivers.

