Resources
Video resources

A story of wonder
A testimonial from Ashley, a mother and caregiver, about her daughter Autumn and their journey from diagnosis to treatment with RETHYMIC and beyond.

How RETHYMIC is manufactured
A look at each stage of the engineering process of RETHYMIC.
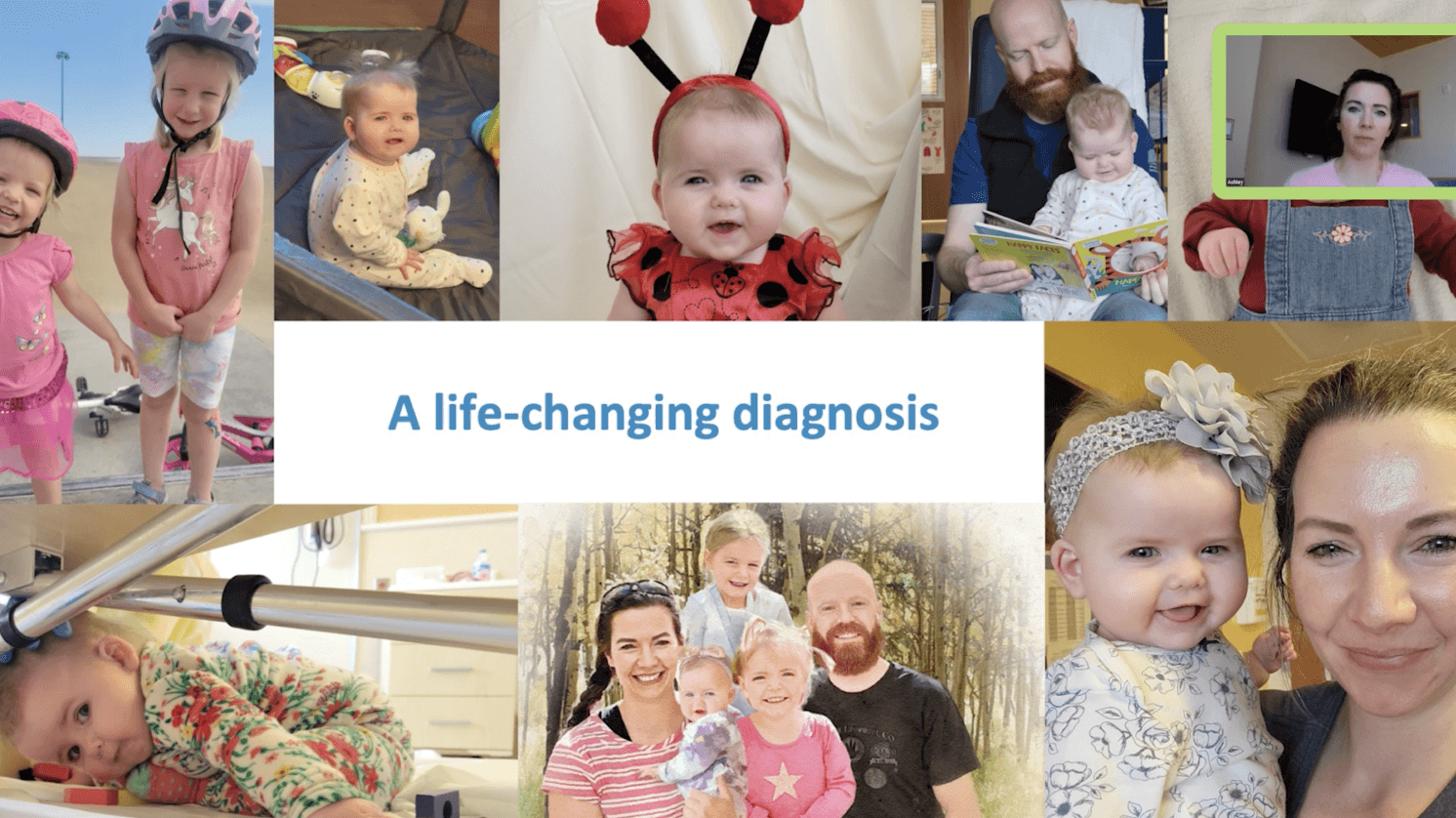
Understanding the congenital athymia patient journey
An edited recording of a webinar featuring a caregiver and a healthcare provider discussing the treatment journey and their partnership. This webinar was originally available as part of the Enzyvant CONNECT support program, which is now known as RETHYMIC Connect™.

Congenital athymia and life after treatment
A recorded webinar featuring Dr John Sleasman, providing his perspective on the congenital athymia treatment journey. This webinar was originally available as part of the RETHYMIC Connect support program.
Resources for your practice

Understanding Congenital Athymia
A guide to congenital athymia, how it’s diagnosed, and supportive care.

A Guide to RETHYMIC
An informative brochure with study results, details about the engineering process, and more.

Patient Case Study
A case study following a hypothetical patient with congenital athymia through the RETHYMIC treatment journey.

Understanding the Path to a Congenital Athymia Diagnosis
An informative brochure detailing each of the steps involved in confirming a diagnosis of congenital athymia.
Resources for your patients’ caregivers

Understanding and Living With Congenital Athymia
A guide to congenital athymia, from diagnosis to creating a care plan, specifically for caregivers.

A Caregiver’s Guide to RETHYMIC
A guide to help caregivers better understand what RETHYMIC is, how it may help their child with congenital athymia, and more.

The Road to RETHYMIC
A guide to help caregivers navigate the different steps of the congenital athymia treatment journey, from diagnosis to receiving RETHYMIC and beyond.
Resources for thymus tissue donation
Thymus tissue donation for RETHYMIC production and for related research is only available at select hospitals in the United States.

A Potentially Life-Changing Donation
A testimonial from Chelsy, a mother and caregiver, about her son Gabe, his journey with congenital athymia, and the importance and process of donating thymus tissue.

Thymus Tissue Donation
An informative brochure about congenital athymia and thymus tissue donation that describes the donation process and its potential impact.
Patient organizations:
There are several organizations for patients with immune system diseases that provide valuable support and education.

Jeffrey Modell Foundation is a global patient organization devoted to early and precise diagnoses, meaningful treatments, and ultimately, cures for primary immunodeficiencies (PIs).

Global Genes is dedicated to eliminating the burdens and challenges of rare diseases for patients and families.

The Immune Deficiency Foundation is dedicated to improving the diagnosis, treatment, and quality of life of people affected by PIs by fostering a community empowered by advocacy, education, and research.

The National Organization for Rare Disorders is a patient advocacy organization dedicated to individuals with rare diseases and the organizations that serve them.



